
ABOUT
With over 12 years of experience at GMPharma, I am dedicated to delivering high-quality compliance solutions. My passion for excellence and commitment to clients and patients set me apart. I ensure that my Validation and Project Management services meet the highest industry standards, providing tailored solutions to support your success.
MISSION
I specialise in assisting small to medium businesses to decide on the right pathway to launch their products without needing to invest heavily right from the start. By effectively managing projects, adhering to all regulatory requirements, and optimizing the Quality Management System (QMS), I am dedicated to delivering the highest quality outcomes for my clients.

SERVICES
At GMPharma, I’m your go-to expert for Qualification and Validation. I use the latest risk-based Computer System Validation (CSV) methods to make sure your systems are ready for inspections by the TGA, FDA, EMA, MHRA, and other regulators. With a solid scale-up and development background across the pharmaceutical, biotech, medical device, clinical research, and software industries, I’m here to help you stay on track with compliance.
GMP Readiness and Audits/Certification
I offer full-on audit services in various GxP areas worldwide, including gap analysis and risk assessment, using my team’s diverse language skills to save you time and money. My auditors are well-versed across the entire development and regulatory landscape, covering GMP readiness in compliance with TGA, FDA, MHRA, PIC/S, EU, ISO13485, ISO14644 and ISO9001.Quality Management Systems (QMS/eQMS)
I work with your team to provide hands-on support for QMS software and tools (Change Controls, CAPA, NCR, etc) within your quality system requirements and standards With years of experience in consulting and operational roles, I help you navigate the process and defend your CAPA systems to regulators while optimising your program.
Other Services
- Validation Consultancy
- Vendor Assurance/Supplier audits systems
- Facility/Cleanroom Design and Setup
- Audit Findings and Responses
- Technical Writing

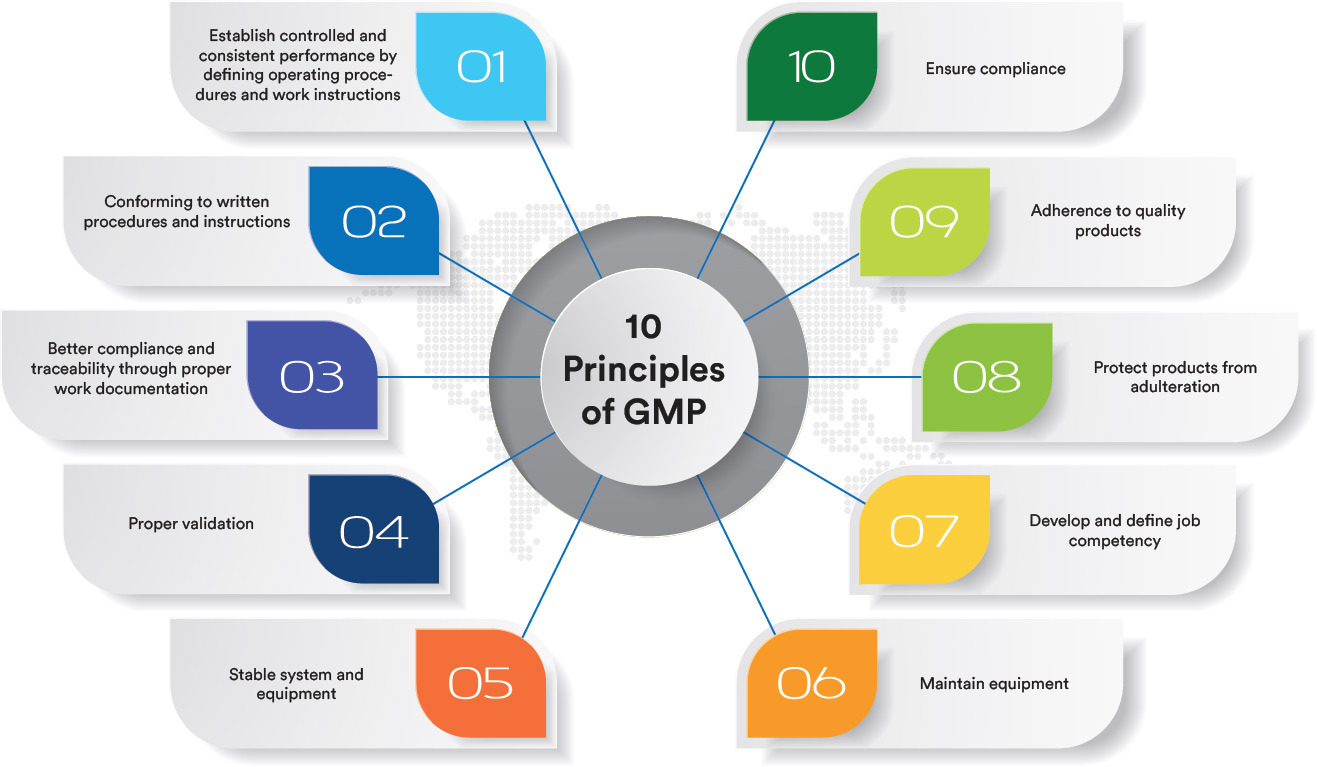

WHY GMPharma?
GMPharma leverages cutting-edge tools and technologies to stay at the forefront of the pharmaceutical and biotechnology industries. With a commitment to innovation, investment in advanced systems, and continuous expansion of a global network, GMPharma provides top-tier quality services while reducing environmental impact. Focused on excellence in every aspect of GMP compliance, GMPharma goes beyond expectations to deliver what truly matters to clients. Quality excellence is in GMPharma’s DNA.